October 3, 2022
Blog
Overcoming clinical trials labeling challenges in the era of decentralization
Bob Bowdish
Senior Account Executive – Clinical Trials
Share
Decentralization is the central theme behind many of the transformative changes currently taking place across the clinical trials industry; from the continued growth of Decentralized Clinical Trials (DCTs) to the shift away from global hubs to regionalized supply chains. Sponsors and their partners are taking a more distributed approach to all aspects of their operations, and it is widely expected that this trend will continue.
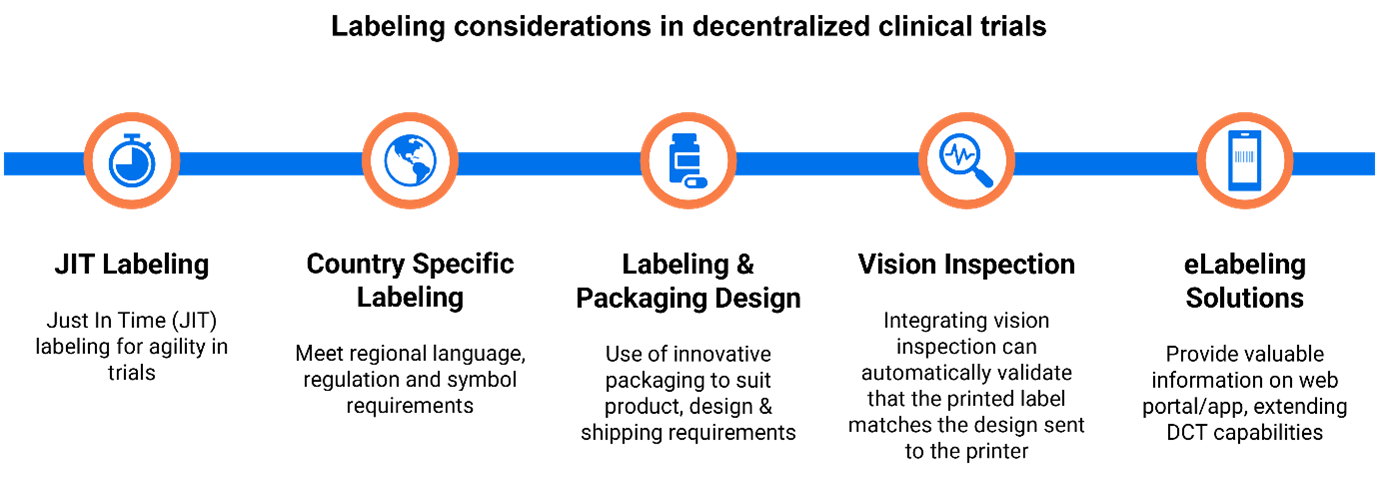
Understandably, these radical paradigm shifts are resulting in a myriad of logistical challenges, including many relating to labeling. In the midst of this shakeup, organizations are taking the opportunity to review their approach to labeling, exploring opportunities for automation, and aiming to dispense with inherently risky and time-consuming manual processes
Decentralized Clinical Trials (DCTs)
Before the Covid-19 pandemic, less than a third of sponsors were operating DCTs, often being discouraged by the logistical hurdles, however, the need to continue research combined with technological advances and the availability of mobile clinical teams meant they were willing to experiment with novel approaches to delivering clinical trials.
Now, as we “return to normality”, appetite for the use of DCTs, both with and without Direct to Patient (DTP) dispensing, remains high. It is anticipated that the total number of trials with decentralized aspects will increase by 28% in 2022, representing a 93% increase from 2020 levels, and surveys of industry figures show that 76% of respondents plan on using DTP in the future.
For example, DCTs allow for a larger pool of candidates, especially valuable for orphan drug studies which face considerable recruitment challenges. One recent survey of 1,183 cancer patients and survivors revealed that while only 18% had participated in a trial, 77% would have been willing to join if it had been as easy to access as their regular care in terms of distance and frequency of visits.
Changes to supply models
There are two key drivers to an increased usage of Just-In-Time (JIT) or On Demand supply:
- Flexibility: Adaptive trial designs have proved helpful in reducing drug development times and are now being widely used. JIT or On Demand supply allows for changes to trial protocols can be actioned at speed and without the need for re-labeling, in addition to making it easier to incorporate additional countries (invariably with country-specific labeling requirements) into a study.
- Cost: There is the potential to significantly reduce the cost of trials, by further reducing the wastage of IMPs and comparators – this is particularly important with the continued growth of expensive biologic studies. While predictive modeling has allowed organizations to cut wastage to less than ten percent, this still potentially equates to millions of dollars of unnecessary expense than when taking a demand-led approach.
Decentralized supply chains
In addition to assisting with the expanded use of DCTs, the shift to regionalized supply hubs is being spurred by demand for a more flexible and resilient supply chain in the wake of disruption caused by the pandemic and the specter of future issues fueled by geo-political strife.
Furthermore, under pressure to deliver a swift return on investment, sponsors are also demanding that IMPs reach trial participants at unprecedented speed, and the short shelf-life of some IMPs necessitates the use of demand-led production & print models.
Label content needs to be carefully coordinated across these distributed production and delivery sites; consistency is critical to maintaining trial blinding and it is difficult to achieve this with manual processes that are both time-consuming and risky.
Opportunities for CROs, CMOs, and CDMOs
As the clinical trial industry embraces decentralization, there is an opportunity for organizations to embrace the modern integrated approach to labeling so that they can deliver the agile supply chain demanded by sponsors, eliminating costly and labor-intensive manual processes that can easily lead to production bottlenecks.
A purpose-built cloud-based solution for clinical trial label management can serve as a single source of truth for regulated label content, providing a consistent environment across a distributed global supply chain. Loftware Prisym 360 integrates specialist functionality to support compliance, including a regulatory rules engine and support for vision inspection while providing interoperability with PLM and MES systems and boasting on-demand print capability.
Download our new report ‘Embracing Decentralization in Clinical Trials’ to learn more about the impact of decentralization on the clinical trials industry and how a clinical labeling solution can help organizations overcome some of the associated logistical challenges.
